[1] Fernandez, Juliette, et al. "Transportin-1 binds to the HIV-1 capsid via a nuclear localization signal and triggers uncoating." Nature microbiology 4.11 (2019): 1840-1850.
[2] Vargas, Jessica Y., et al. "The Wnt/Ca2+ pathway is involved in interneuronal communication mediated by tunneling nanotubes." The EMBO journal 38.23 (2019): e101230.
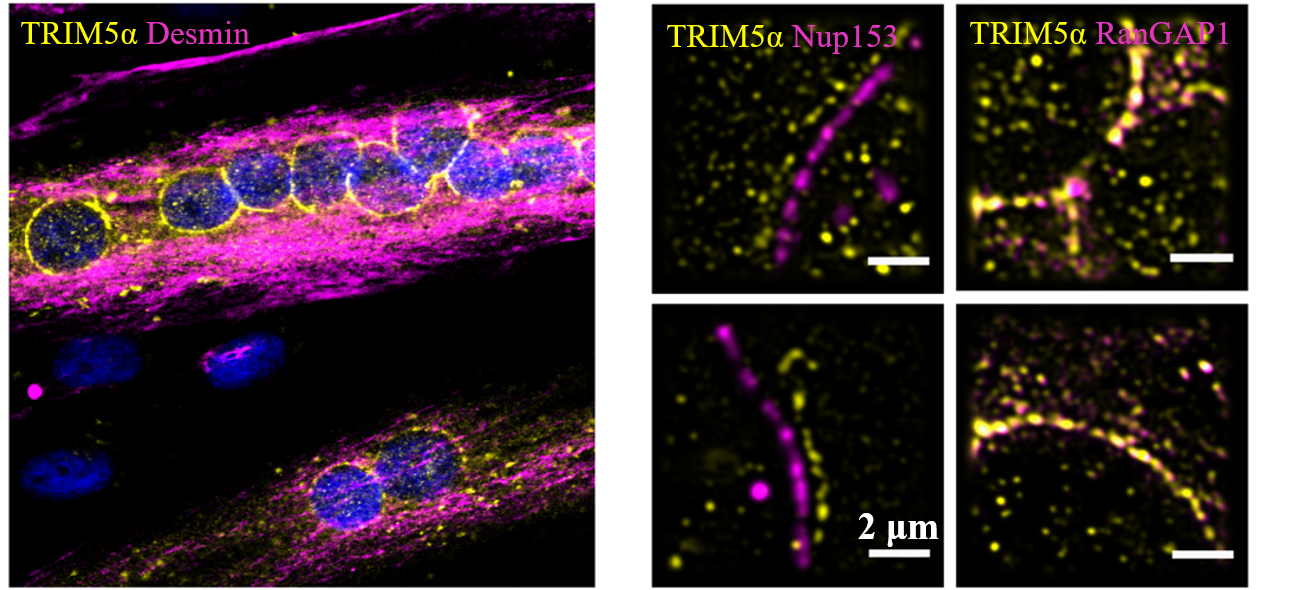
[3] Maarifi, Ghizlane, et al. "RanBP2 regulates the anti-retroviral activity of TRIM5α by SUMOylation at a predicted phosphorylated SUMOylation motif." Communications biology 1.1 (2018): 1-11.
[4] Garita-Hernandez, Marcela, et al. "Optogenetic light sensors in human retinal organoids." Frontiers in neuroscience 12 (2018): 789.
[5] Getz, Angela M., et al. "Tumor suppressor menin is required for subunit-specific nAChR α5 transcription and nAChR-dependent presynaptic facilitation in cultured mouse hippocampal neurons." Scientific reports 7.1 (2017): 1-16.
[6] Portilho, Débora M., Roger Persson, and Nathalie Arhel. "Role of non-motile microtubule-associated proteins in virus trafficking." Biomolecular concepts 7.5-6 (2016): 283-292.
[7] Pagliuso, Alessandro, et al. "A role for septin 2 in Drp1‐mediated mitochondrial fission." EMBO reports 17.6 (2016): 858-873.
[8] Fallet, Clement, and Gabriel Y. Sirat. "Achromatization of conical diffraction: application to the generation of a polychromatic optical vortex." Optics letters 41.4 (2016): 769-772.
[9] Fallet, Clement, et al. "Accurate axial localization by conical diffraction beam shaping generating a dark-helix PSF." Single Molecule Spectroscopy and Superresolution Imaging IX. Vol. 9714. International Society for Optics and Photonics, 2016.
[10] Fallet, Clement, Arvid Lindberg, and Gabriel Y. Sirat. "Generating 3D depletion distribution in an achromatic single-channel monolithic system." Single Molecule Spectroscopy and Superresolution Imaging IX. Vol. 9714. International Society for Optics and Photonics, 2016.
[11] Fallet, Clément, et al. "A new method to achieve tens of nm axial super-localization based on conical diffraction PSF shaping." Single Molecule Spectroscopy and Superresolution Imaging VIII. Vol. 9331. International Society for Optics and Photonics, 2015.
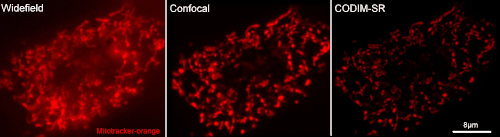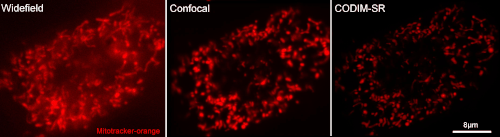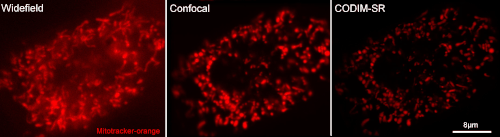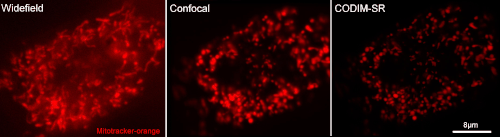
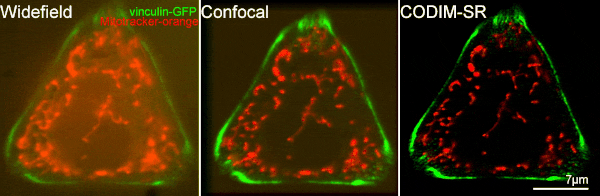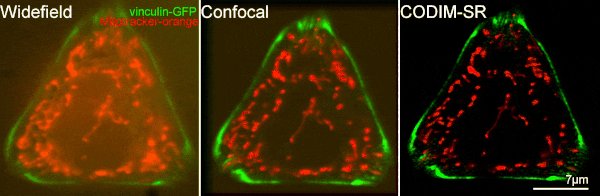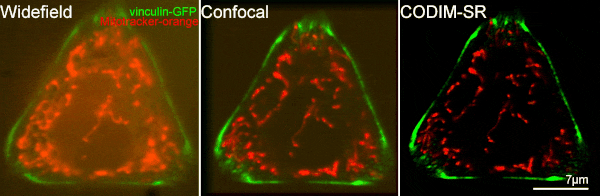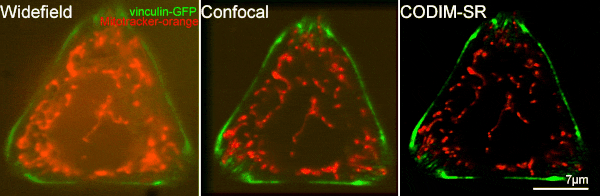
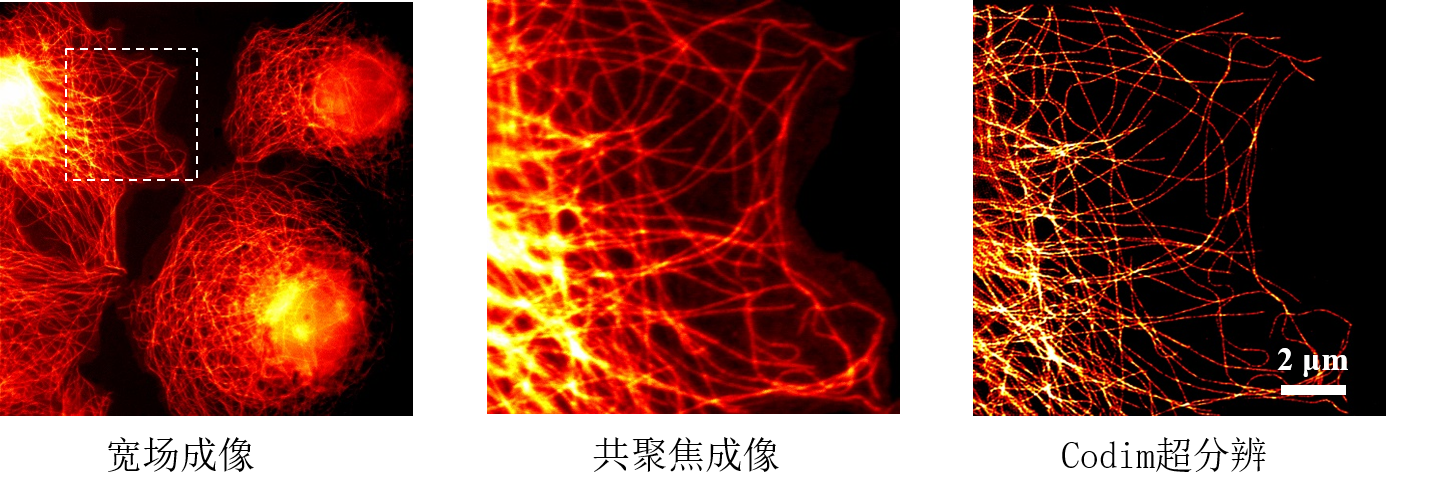
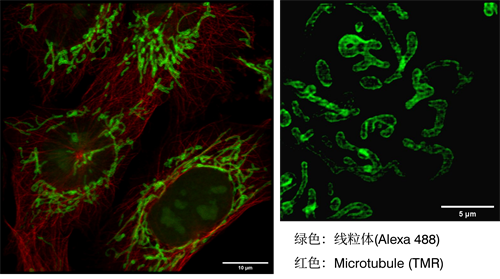
[12] Caron, Julien, et al. "Conical diffraction illumination opens the way for low phototoxicity super-resolution imaging." Cell adhesion & migration 8.5 (2014): 430-439.
[13] Fallet, Clément, et al. "Conical diffraction as a versatile building block to implement new imaging modalities for superresolution in fluorescence microscopy." Nanoimaging and Nanospectroscopy II. Vol. 9169. International Society for Optics and Photonics, 2014.
[14] Rosset, Sybille, Clement Fallet, and Gabriel Y. Sirat. "Focusing by a high numerical aperture lens of distributions generated by conical diffraction." Optics letters 39.23 (2014): 6569-6572.